Artificial Creation of Floral Scents
ABSTRACT
The artificial synthesis of esters is an important process to know and understand in many industries, one of them being candle making. On behalf of the Sojeii Candle Company, the purpose of this series of Fischer esterification experiments is to find which combination of acid and alcohol creates the scent of rose, lavender, and primrose. The molecular formula of these created esters will also be determined so that others who replicate this series will know exactly how much of each reactant is needed to make an exact amount of ester.
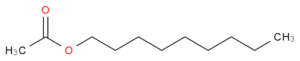
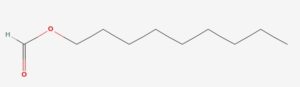
As of now, there is only one process of esters know to make the scent of a banana, which is by the esterification of isopentyl acetate, C7H14O2. The following will demonstrate the esterification of nonyl acetate, C11H24O3 (rose), nonyl formate, C11H24O5 (lavender), and nonyl propionate, C12H27O5 (primrose).
INTRODUCTION
Esters are created from the reaction between a carboxylic acid and an alcohol. In this process, one -OH (hydroxyl) group is replaced with an -O-alkyl (alkoxy) group. When a carbon bonds with an oxygen atom, depending on the length of the reaction, create scents of various kinds, such as apple. This is so, because these esterification reactions occur naturally within fruits and vegetables and therefore by artificially inducing these reactions, scents can be replicated in the lab. Many companies already use this idea of synthesizing esters to create fruity scents, such as pineapple formed from ethyl propionate. However, these experiments will enable the spectrum of scents to extend further into the floral section.
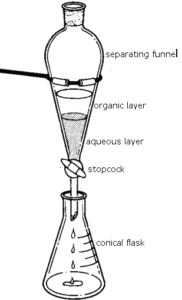
An unaffected ester experiment normally takes a few hours, just like how naturally in nature, it takes fruits and vegetables time to mature, the synthesis of the ester scents too need time. In order to speed up the reaction, aside from the two reactants, acetic acid and isopentyl alcohol, there is the addition of sulfuric acid, a catalyst, whose purpose is to speed up the rate of reaction. Just a catalyst isnt enough to speed up the reaction so the use of heat also speeds up the rate of the reaction. Esters are insoluble in water so adding water does not affect the product created and in fact separate the excessive reactants from the products because the, acetic acid and isopentyl alcohol are soluble. Therefore, by adding water into the separating funnel, the excess reagents coating the ester is washed away. The density of the ester is less than that of the aqueous solution (the water, acetic acid and isopentyl alcohol) therefore it will settle at the bottom of the separating funnel.
A limiting reagent is the reactant in an experiment that determines the most amount of product that can be created. To determine which reagent is the limiting reagent, varying among of the reagents involved should be used in each trail of the experiment and by finding the ratio between the two will provide the answer to this question and determine the molecular formula of the final product. The finals step of the synthesis is to repeat the trails but without the use of the hot water bath or the boiling chips and the purpose was to allow for the reaction to occur at its natural rate of reaction, proving that the hot water bath and the boiling chips had no effect on the experiment itself.
DIAGRAM

METHODS
-
Synthesizing the Ester
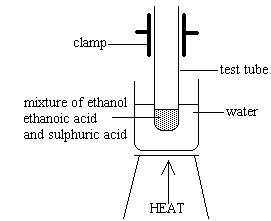
Create a hot water bath by filling a 250 mL beaker halfway and bring it to a temperature between 80oC-85oC on a heat plate. The thermometer should be inside the beaker, held by a clasp. While heating, in a 100 mL round bottom flask, add 35 mL of glacial acetic acid (0.200M) and 25 mL of nonanal alcohol (0.500M). Swirl the flask until mixed. Add in 5 mL of concentrated sulfuric acid and swirl the flask until the solution is homogeneous. Place the flask in the water bath, suspending it so it doesn’t touch the bottom of the beaker. Ensure that the water does not boil and is remained at a constant temperature between 80oC-85oC. Add boiling chips so that the heating process will occur at a slower rate to ensure that the scent that is sought after is achieved. Over boiling the solution will cause a different scent to be released. Allow the mixture to be cooled after heating for 30 minutes.
Transfer the solution to a separating funnel along with 20 mL of water. Place the stopper on and shake the funnel. Allow some time for the layers to separate afterwards. Place an Erlenmeyer flask below the stopper and open the stopcock so that the aqueous solution drips through. The remaining layer is the ester that produces the fragrant scent, which would be nonyl acetate. Weigh the amount of nonyl acetate created. Repeat this process five more times, for a total of six trails per each, using the following guide.

These steps are to be repeated with formic acid (0.200M) to create nonyl formate, and propionic acid (0.200M) to create nonyl propionate. Repeat the entire experiment twice. Both times without the use of the hot water bath or the boiling chips.
-
Determining the Molecular Formula
Determine the number of moles of glacial acetic, formic, and propionic acid using molarity formula. Repeat this step to determine the number of moles of nonanal alcohol. Input these numbers into a spreadsheet on excel, creating a data table. Insert a scatter plot and select your data. This data chart will yield two lines, one with an increasing slope and one with a decreasing slope. To create the graph with the increasing slope, select the first three rows of data, x-values being the mols of the respective acid and the y-axis being the grams of ester that was produced. Repeat this step except with x-value and y-values from the last three rows in the data sheet. This will provide a graph with two sets of points, in which a trendline will be added for each set of points. Select “Display Equation on Chart” and by doing this, a best fit line will be created. Set the equations equal to each other solve for the variable, which in this case would indicate how much of the nonanal alcohol (C9H20O) was used during each reaction. The final step is to determine the molecular formula between the respective acid and nonanal acid by finding the ratio between the two reactants. The ratio represents the subscripts of the reactants.
DATA
-
Nonyl Acetate
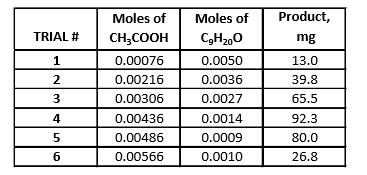

-
Nonyl Formate
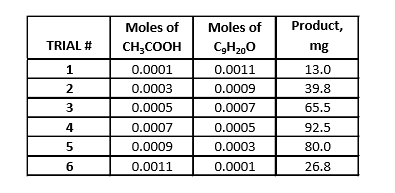

-
Nonyl Propionate


CONCLUSION
Before these experiments were carried out, there were no esters created using any of the nonyl- alkyl hydrocarbon group. Through this set of experiments, one can assume that for the esterification of nonyl octanoate, nonyl salicylaldehyde, and nonyl hexanoate will too produce a floral scent. Therefore, it can be concluded that when the nonyl- alkyl hydrocarbon group reacts with any type of carboxylic acid, the interaction between the hydroxyl and oxygen atoms imitate that of what is created when a flower secretes their aroma either form their scent glands or due to their natural ability to create chemical reactions within the organism itself.
CITATIONS
INTRODUCING ESTERS, Reactions of the Group 2 Elements with Water. (n.d.). https://www.chemguide.co.uk/organicprops/esters/background.html (accessed March 3, 2019).
Making scents: The aromatic world of flowers, Scienceline. (2013). https://scienceline.org/2013/01/making-scents-the-aromatic-world-of-flowers/ (accessed March 3, 2019).
J. Pernaa, M. Aksela, Learning Organic Chemistry through a Study of Semiochemicals, Journal of Chemical Education. 88 (2011) 1644–1647. doi:10.1021/ed900050g. Infographic: Table of Esters and their Smells, James Kennedy. (2013). https://jameskennedymonash.wordpress.com/2013/12/13/infographic-table-of-esters-and-their-smells/ (accessed March 3, 2019).
J. Stubbings, Synthesis, Isolation and Purification of Esters in a Direct Esterification Reaction Chemistry Tutorial, Chemistry Tutorial : Aspirin (Acetylsalicylic Acid). (n.d.). https://www.ausetute.com.au/esters.html (accessed March 3, 2019).



